


















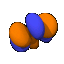
Electron orbitals are the probability distribution of an electron in a atom or molecule. The shape of the orbital depends on the quantum numbers associated with the particular energy state. These are n, the principal quantum number, l, the oribital quantum number, and m, the angular momentum quantum number.
| n=1,l=0 | n=2,l=0 | n=2,l=1 | n=3,l=0 | n=3,l=1 | n=3,l=2 | n=4,l=0 | n=4,l=1 | n=4,l=2 | n=4,l=3 | |
| m=0 |  |
 |  |
 |  |  |
 |  |  |  |
| m=1 |  |
 |  |
 |  |  |
||||
| m=2 |  |
 |  |
|||||||
| m=3 | 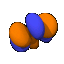 |
A note about the drawings: The blue color indicates a positive phase, while the orange color indicates a negative phase. The colors become important when molecular orbitals are computed.
The electron orbitals presented here represent a volume of space within which an electron would have a certain probability of being based on particular energy states and atoms. For example, in a simple lowest-energy state hydrogen atom, the electrons are most likely to be found within a sphere around the nucleus of an atom. In a higher energy state, the shapes become lobes and rings, due to the interaction of the quantum effects between the different atomic particles.
 These shapes continue on infinitely, getting ever more lobes or rings on them. Although the l=0, m=0 orbitals look like simple spheres, regardless of n value, this is not actually the case. To the right is a cutaway of a 4s0 (n=4, l=0, m=0) oribital, showing that it is really concentric spheres.
These shapes continue on infinitely, getting ever more lobes or rings on them. Although the l=0, m=0 orbitals look like simple spheres, regardless of n value, this is not actually the case. To the right is a cutaway of a 4s0 (n=4, l=0, m=0) oribital, showing that it is really concentric spheres.
 These pictures have been of electron orbitals associated with a single atom. Molecules can become much more complicated. When two atoms are within a certain proximity of each other, the orbital probabilities can either reinforce each other or cancel each other out. If the phase is the same sign (the same color), the probabilities are reinforced. To the right is a picture of the bonding orbit for H2O (water).
These pictures have been of electron orbitals associated with a single atom. Molecules can become much more complicated. When two atoms are within a certain proximity of each other, the orbital probabilities can either reinforce each other or cancel each other out. If the phase is the same sign (the same color), the probabilities are reinforced. To the right is a picture of the bonding orbit for H2O (water).