AP Physics Lab
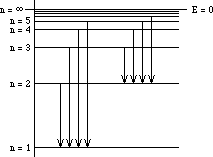
Energy levels of the Hydrogen Atom
Obj: Determine the photon energy and quantum number of
electrons returning to n = 2 (Balmer Series).
Materials: H2 gas tube, spectrometer
Methods & Analysis
(Note: run the spectrum tube for no more than 30 seconds with a 30 second cooling period.)
1. Estimate the of the red, blue, and violet bright lines to the nearest 100 nm. Compare with the actual values.
Note: 5000 Angstroms = 500 nm and 1 nm = 1 X 10-9 m
2. Calculate the energy of the photon for each color in Joules and eV using the actual values.
3. Find the difference in energy between the photon and the n = 2 energy level (3.40 eV).
4. Determine the quantum number from the reference table and verify with the equations in your textbook where R is the Rydberg Constant.
5. Which color has the highest energy? the shortest ?
Back to the Brockport High School Science Department